Pioneering Sustained
NITRIC OXIDE
Delivery Systems
Harnessing the power of Nitric Oxide (NO) for anti-microbial, anti-inflammatory, and anti-thrombotic therapies.
Sustained Nitric Oxide (NO) Release to Prevent Infection
Nytricx is revolutionizing infection prevention and vascular health with technology mimicking the body’s natural release of nitric oxide (NO). A game-changing antimicrobial solution for wound care and catheter infection and thrombosis — addressing a huge expanding global market.

Nytricx Focus
- Wound Infections: Millions face non-healing wounds, amputations, and severe complications.
- Catheter-Associated Infections: 800,000 US hospital infections annually, over 40,000 annual deaths.
- Current Solutions Often Fail: Antibiotic resistance is a growing threat.
The Nytricx Breakthrough Solution
Nytricx products release controlled levels of NO, the body’s natural antimicrobial, locally preventing infection and saving lives.
Wound Dressing (with or without TXA)
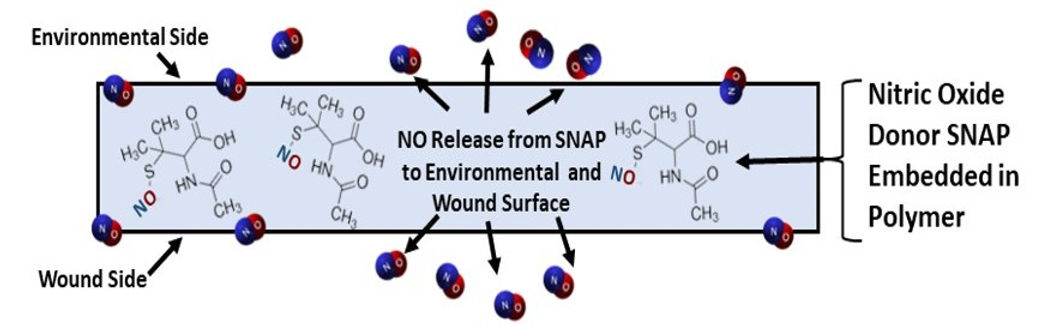
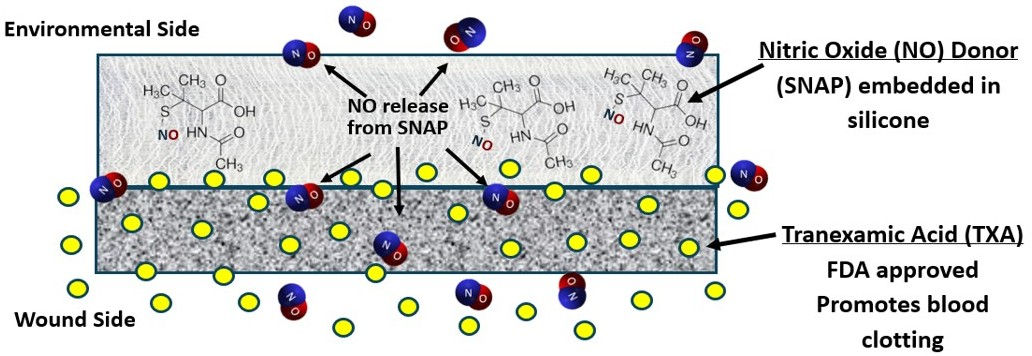
Nytricx advanced wound patch continuously releases nitric oxide (NO), the body’s natural defense molecule, killing harmful bacteria, reducing inflammation, and promoting healing. Thin, flexible, and easy to apply, the patch offers a breakthrough solution for chronic and traumatic wounds, without the use of antibiotics or risk of resistance.
Catheter Inserts
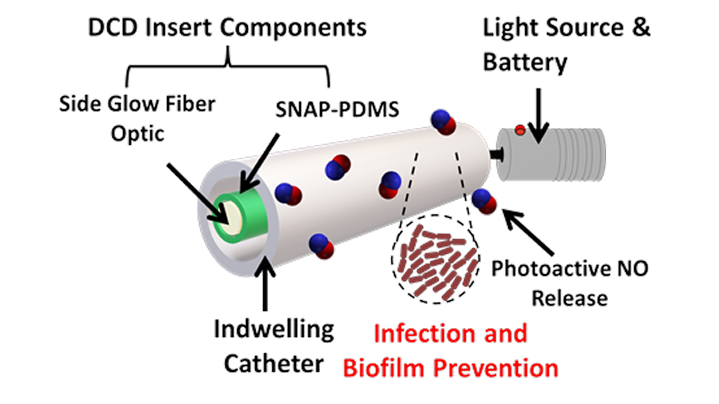
Nytricx disposable catheter disinfection (DCD) inserts release nitric oxide (NO) into the inner lumen of standard central venous catheters (CVC) and urinary catheters, preventing dangerous infections and thrombosis (CVCs). The fiber optic-based insert technology allows real-time control of NO release offering a smarter, safer way of catheter maintenance without the use of antibiotics.
A Multi-Billion Dollar Global Market
- Multi-billion dollar market (wounds + catheters).
- 50–60% of diabetic wounds get infected.
- 1.7M US hospital-acquired infections annually.
- NO-releasing technology: no resistance, no systemic antibiotics.
Nytricx: A University of Georgia Start-up with $6.2M Non-Dilutive Funding
Nytricx, Inc. nitric oxide research has roots in the development and testing facilities at the University of Georgia, College of Engineering Research Laboratories of Drs. Hitesh Handa and Elizabeth Brisbois
Seeking $10M Series A for Clinical Development
- $2M Finalize Formulation & Safety Testing
- $4M Manufacturing & Regulatory
- $4M Phase II Clinical Studies
Contact Us
Ronald Shebuski,
PhD, President and CEO